Updated 12/30/2022
We investigate the molecular mechanisms and pathophysiological roles of mitochondrial Ca2+ transport, signaling, and homeostasis.
Mitochondrial Ca2+ controls the life and death of mammalian cells. It regulates intracellular Ca2+ signals, modulates ATP synthesis in mitochondria, and plays key roles in programmed cell death. Perturbation of mitochondrial Ca2+ homeostasis has been linked to progression of neurodegenerative diseases (e.g., Alzheimer's) and injury of cardiac muscles during myocardial-ischemia reperfusion.
We ask the following questions:
1. What are the molecular mechanisms of mitochondrial Ca2+ channels and transporters?
2. How do mitochondrial Ca2+ transport proteins control mitochondrial Ca2+ homeostasis and signaling to regulate physiological processes?
3. How could we treat pathological conditions by manipulating mitochondrial Ca2+ transport?
Our research extends from molecular to cellular levels.
Project #1: Molecular mechanisms of the mitochondrial calcium uniporter
The uniporter is a multi-subunit Ca2+ channel complex that imports cytoplasmic Ca2+ into the mitochondrial matrix. We combine membrane-protein purification, biochemical analyses, functional reconstitution, electrophsyiology, and structural biology to investigate how the uniporter operates as a molecular machine using the principles of physics and chemistry.
Our achievements
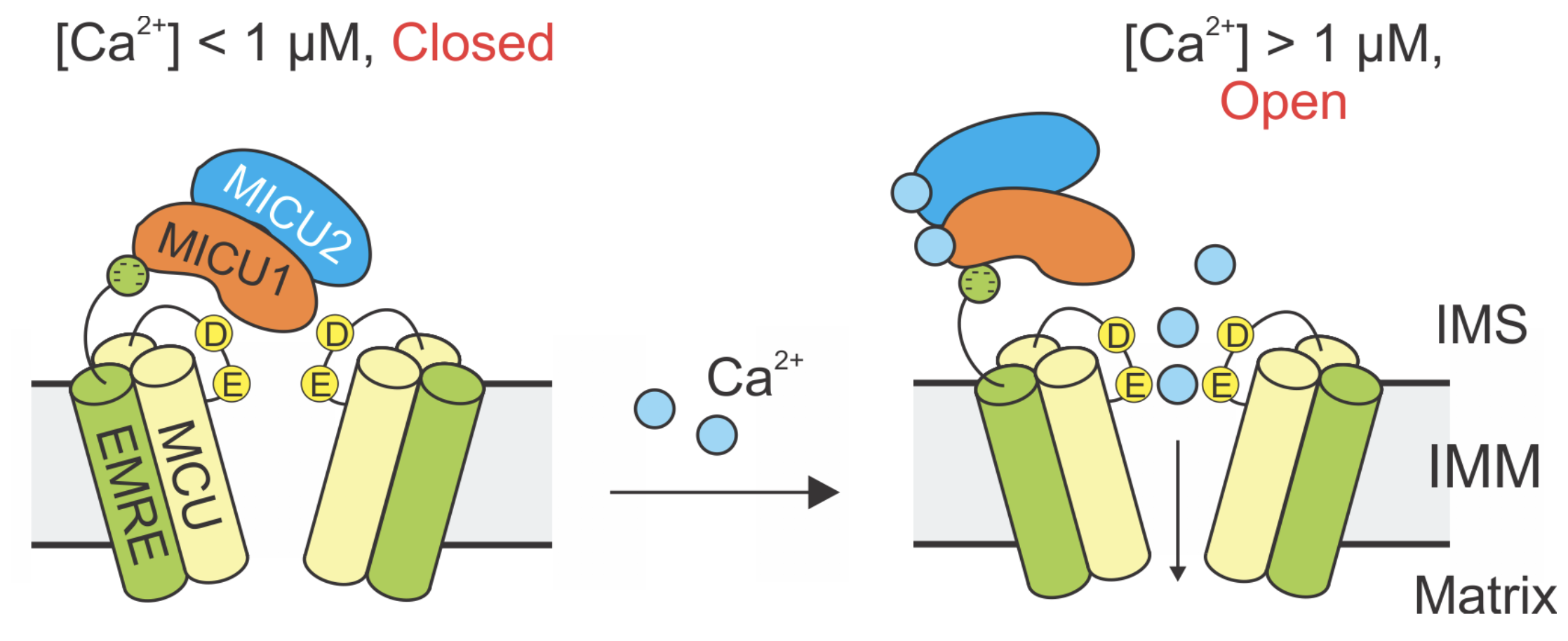
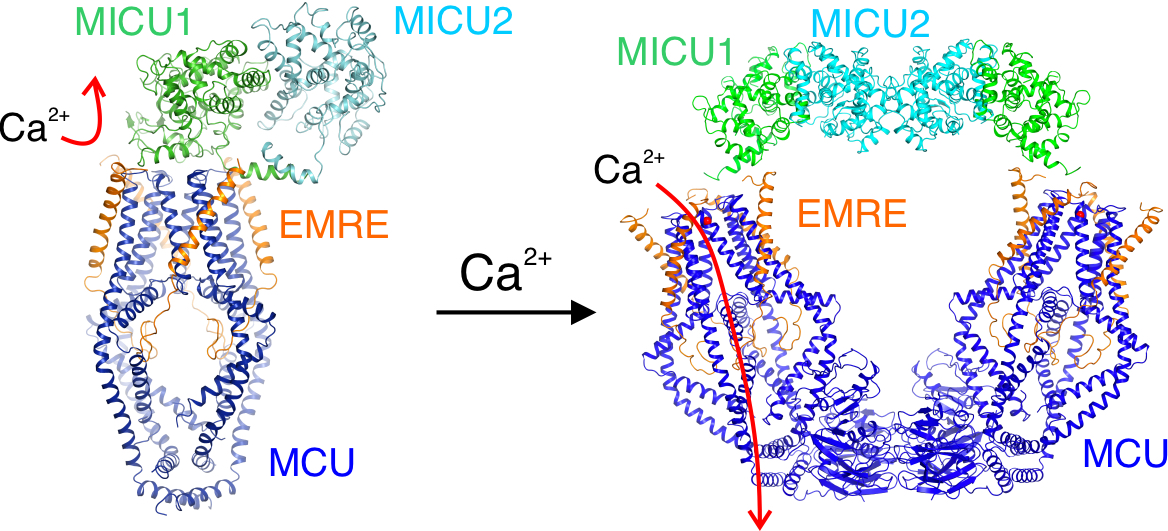
A structural-functional studies with our collaborators at Stanford produced a molecular model that explains how intracellular Ca2+ signals can control the opening and closing of the uniporter. Link
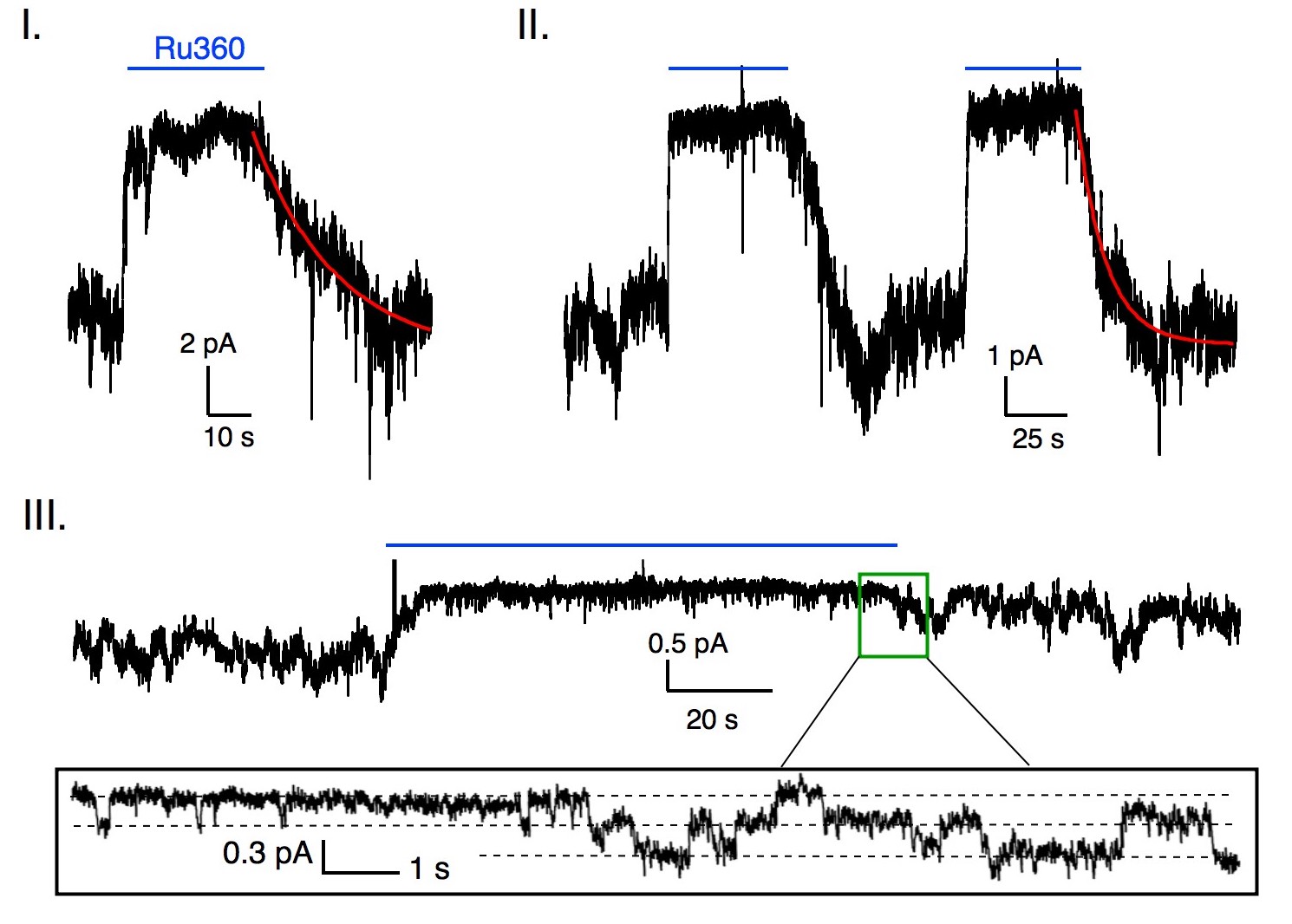
Patch-clamp electrophysiology has been the gold standard of ion-channel analysis, but applying this approach to the uniporter is difficult because the mitochondrial membrane is small.
We have overcome this problem by engineering uniporter proteins to travel to cell membranes. This allows us to efficiently obtain macroscopic and single-channel recordings. Link
Project #2: The roles of mitochondrial Ca2+ transport in physiology
We investigate how a network of proteins act together to achieve mitochondrial Ca2+ homeostasis, and how perturbation of their functions impact cellular physiology. Live cell imaging, induced pluripotency stem cells, animal models, and CRISPR genome editing methods are combined to address our research questions.
Achievements
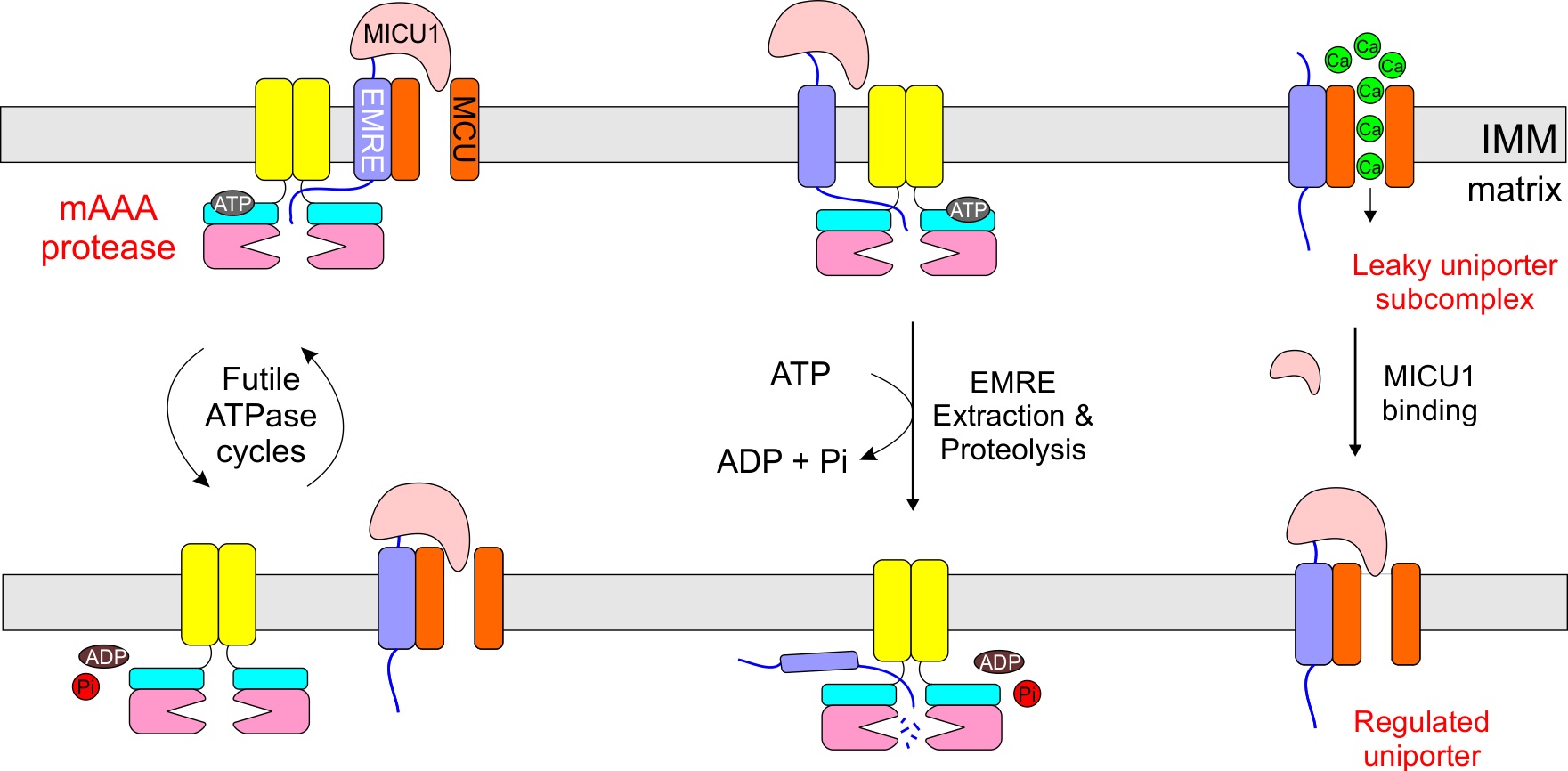
We identified a protease pathway, in which mitochondrial AAA proteases AFG3L2 and SPG7 use the energy of ATP hydrolysis to remove an excessive uniporter subunit. Perturbation of this pathway leads to deregulated uniporter subcompelxes, which can constitutively load calcium into mitochondria to induce cell death. Link
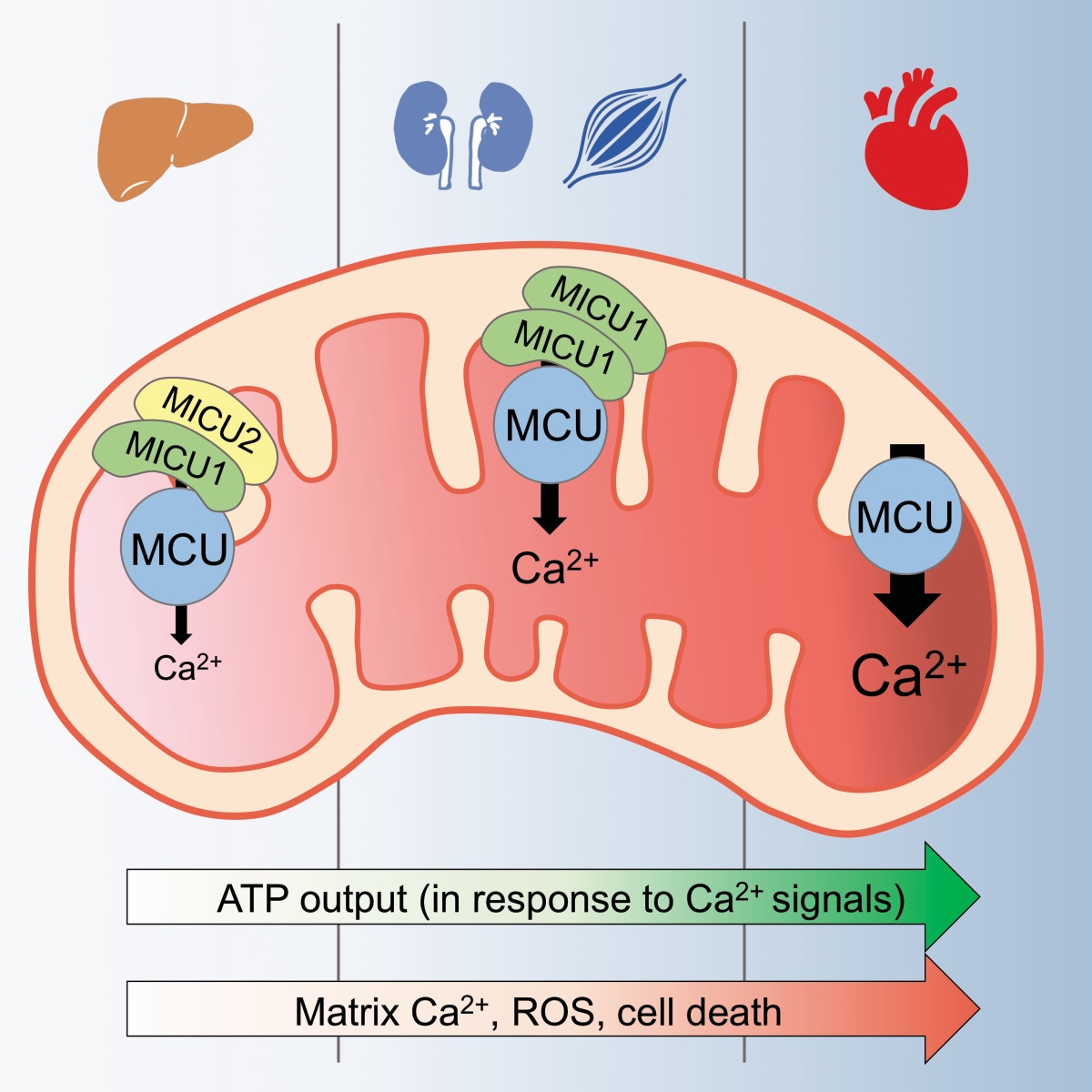
In a recent study, we demonstrated that the uniporter can respond to intracellular calcium signals differently in different types of cells. This is achieved by cells producing different uniporter regulatory subunits. We analyzed the physiological benefits and disadvantages for cells to use different uniporter regulators. This work creates and exciting area of research about how tissue-specific uniporter regulation contributes to unique physiological functions and pathological conditions in different tissues. Link